The factors which influence the life of an elastomeric product fall into two basic categories which can be called "product characteristics" and "ageing processes". There is no chance of retrospectively influencing the product characteristics since these describe the product as it was made and include such variables as:
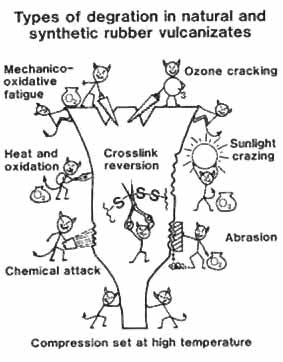
Shelf ageing is basically oxidative degradation and, apart from the obvious influence of oxygen, one must consider the catalytic effects of heat, light, internal and external stresses or strains and pro-oxidant metals. The chemistry of oxidative degradation is, even now, not completely resolved but, in a simplistic form, oxidation of a sulphur vulcanized polyolefin such as natural rubber proceeds via at least one chain reaction sequence which introduces C-C and C-O-O-C crosslinks between polymer chains as well as C-O-O-C rings within the same polymer chain, and another set of chain reactions between oxygen and the sulphur atoms of the crosslinks or pendent groups. These two sequences of chain reactions can result in both chain scission and the formation of additional crosslinks. The reactions between sulphur and oxygen can also, eventually, lead to sulphuric acid formation, a particular problem with ebonites. There is therefore a large range of both sequential (or chain) reactions and competing reactions and that the ones which predominate depend on factors such as the composition of the vulcanizate as well as the influences of heat, light and metal catalysis. In heat ageing we are balancing the rate of reaction of oxygen with the elastomer and the rate of diffusion of the oxygen into the bulk material. If the temperature is relatively low, it has been postulated that for an unprotected vulcanizate diffusion predominates and therefore there is slow oxidation throughout the product, but as the temperature rises, the rate of oxidation increases much more than the rate of diffusion so substantial oxidation occurs on the surface and an oxidized (hard) surface skin is formed. As oxidation continues the chain breakdown may become more significant and the hard surface then softens and turns sticky. To complicate matters further, under certain conditions this order can be reversed and an initially sticky degraded surface can harden with further oxidation. The mechanism for light catalysed oxidative degradation is that the energy of UV light may be sufficient to break a C-H bond and generate a radical species which can then react with oxygen to initiate the same chain reaction sequences as occur in direct oxidation. Two types of protective agents are available, the ultra violet absorbers (UVA's) and the hindered amine light stabilizers (HALS). The UVA's compete with the olefinic double bonds for the available energy and dissipate it harmlessly. It is important to remember that the absorption of light follows Beer's law so even if one has a UVA present it would give no real protection to a material less than about 0.5 millimetres. HALS operate by a different mechanism which is still not completely resolved but is believed to be a radical trap. It thus operates throughout the UV-transparent sample and is often used in conjunction with a UVA. Light-catalysed oxidation can result in an inelastic skin which, as it thickens, cracks in random directions and produces a pattern known as "crazing". In the early stages, in thin sheets, the effect has been called "light stiffening" whilst in highly filled articles the degradation can result in complete loss of the resinified elastomer and one ends up with the "chalking" effect mentioned earlier. This has given rise to comments about 'blooming' inorganic fillers which is mechanistically impossible. Because of the light absorbing properties of black-filled materials, this effect is not normally seen in them. In many real life vulcanizates of reasonable bulk under ambient conditions, it seems that the ingress of oxygen is limited and, apart from the surface few millimetres or so, the bulk rubber remains in excellent condition. Atmospheric ageing differs from shelf ageing in that it is characterized by the attack of ozone on the rubber. It is essential to be aware that this is not just another form of oxygen-induced degradation as the mechanism is quite different, with simple bimolecular ozonolysis of the rubber olefinic double bond being followed by immediate cleavage to give two carbonyl end groups. If the rubber is under any sort of stress this will result in 'atmospheric cracking' in which the cracks are perpendicular to the direction of elongation. As early as 1931 it was shown that 'ozone cracking' was different from 'light ageing' and took place more readily in the night than during the day. The effect of trace metal contaminants such as copper, chromium and iron in any of the compounding chemicals is inevitably to accelerate the rate of degradation although the change in rate cannot be predicted as it depends on many factors, not least the chemical state of the metal. |